


















Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
The Ultimate Guide to Level Calculations in Chemistry: Mastering the Art for Secondary Schools and Colleges

Chemistry can be an exciting and challenging subject, requiring students to delve into the world of atoms, molecules, and equations. One important aspect of chemistry that often perplexes students is level calculations. These calculations involve determining the concentration of a substance in a solution or the amount of substance needed to react completely. In this comprehensive guide, we will break down the concepts, formulas, and strategies essential to mastering level calculations in chemistry.
The Significance of Level Calculations
Level calculations play a crucial role in understanding chemical reactions and determining the precise quantities of substances involved. They are required in various fields such as medicine, environmental sciences, and pharmaceuticals. Whether you aspire to become a chemist, pharmacist, or researcher, a strong foundation in level calculations is essential for success.
The Fundamentals of Level Calculations
Before diving into the nitty-gritty of level calculations, let's review some fundamental concepts that form the basis of this topic:
5 out of 5
| Language | : | English |
| File size | : | 22329 KB |
| Print length | : | 99 pages |
| Lending | : | Enabled |
| Screen Reader | : | Supported |
Moles and Avogadro's Number
In chemistry, a mole refers to 6.022 x 10^23 entities, which could be atoms, molecules, ions, or particles. Avogadro's number represents the number of entities per mole and is a fundamental constant in chemistry. Using Avogadro's number, we can relate the quantity of a substance in moles to its mass or volume.
Molar Mass
Molar mass is the mass of one mole of a substance. It is expressed in grams per mole (g/mol). To calculate the molar mass, we determine the sum of the atomic masses of all atoms in the chemical formula of a substance.
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It allows us to determine the amounts of substances needed or produced during a reaction using balanced chemical equations.
Types of Level Calculations
Level calculations can be categorized into two main types: concentration calculations and reacting mass calculations.
Concentration Calculations
Concentration calculations involve determining the concentration of a solute in a solution. The most common units of concentration are molarity (M) and molality (m). Molarity is defined as the number of moles of solute per liter of solution, while molality is defined as the number of moles of solute per kilogram of solvent.
To perform concentration calculations, we need to know the volume, concentration, and molar mass of the solute, as well as the volume of the solvent. Using these values, we can apply the formula:
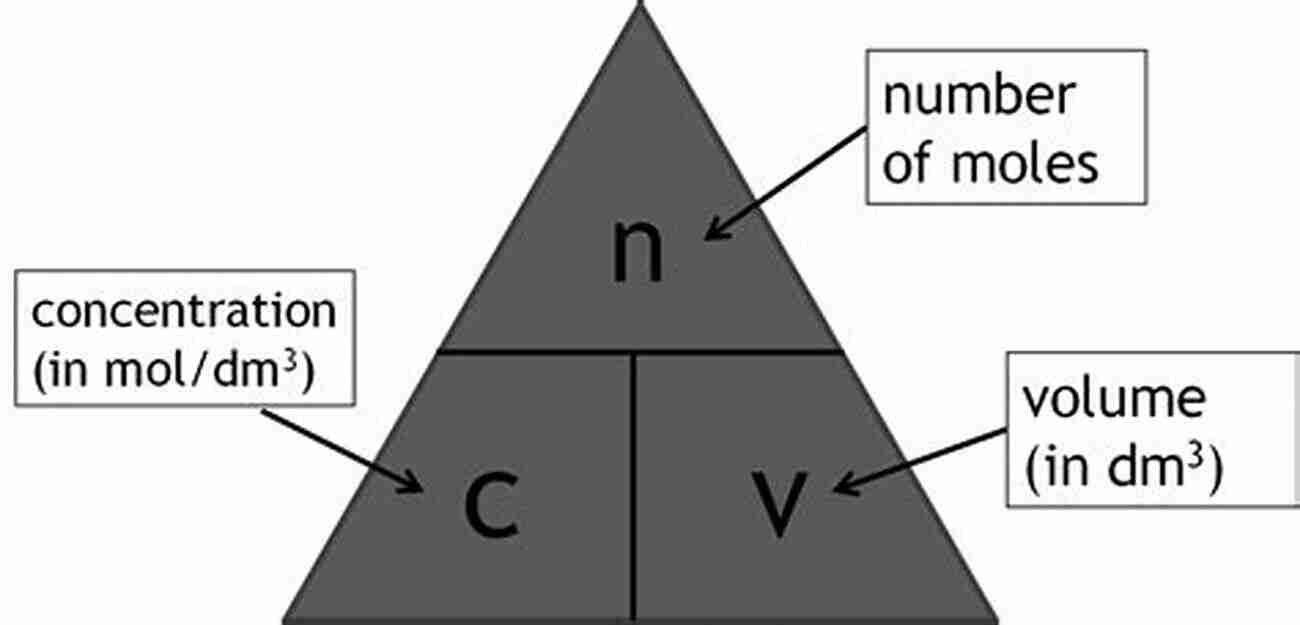
where C represents the concentration, n represents the number of moles of solute, and V represents the volume of the solution.
Reacting Mass Calculations
Reacting mass calculations involve determining the mass of a substance required to react completely with another substance. This type of calculation is especially important when determining the amount of reactants needed to produce a desired product.
To perform reacting mass calculations, we need to balance the chemical equation, determine the number of moles of the known substance, and use stoichiometry to find the mass of the unknown substance.
Step-by-Step Approach to Level Calculations
Level calculations can be daunting, but with a systematic approach, you can conquer them. Follow these step-by-step instructions to tackle level calculations with confidence:
Step 1: Identify the Given Information
Read the problem carefully and identify the relevant information provided, such as the concentration, volume, or mass of the given substances.
Step 2: Determine the Unknown Quantity
Identify the quantity you are asked to calculate, whether it is the concentration, volume, or mass of a substance.
Step 3: Convert Units if Necessary
If the given information is in different units, convert them to a consistent unit system. This step is crucial for accurate calculations.
Step 4: Apply the Appropriate Formula
Select the appropriate formula based on the type of level calculation and the given information. Use the formula to calculate the unknown quantity.
Step 5: Check the Answer
Double-check your calculations and ensure that your answer is reasonable and reflects the problem's context.
Step 6: Practice, Practice, Practice
Level calculations require practice to master. Work through a variety of problems, gradually increasing the complexity, to strengthen your skills.
Tips and Strategies for Success
Here are some tips and strategies to help you excel in level calculations:
1. Understand the Concepts
Ensure that you have a solid understanding of the fundamental concepts, including moles, molar mass, and stoichiometry. Clear concepts will pave the way for successful level calculations.
2. Memorize Important Formulas
Memorize the formulas required for concentration calculations and reacting mass calculations. Being familiar with these formulas will save you time during exams.
3. Use Dimensional Analysis
Dimensional analysis is a powerful tool in chemistry, allowing you to convert units and perform calculations accurately. Master this technique to simplify level calculations.
4. Practice Problem-Solving
The more you practice level calculations, the more comfortable you will become with the concepts and formulas. Seek out additional practice problems, textbooks, and online resources to reinforce your skills.
5. Seek Help When Needed
If you are struggling with level calculations, don't hesitate to seek help from your teacher, classmates, or online tutoring services. Understanding and mastering level calculations is a crucial foundation for your chemistry journey.
Level calculations in chemistry can be challenging, but with practice and a solid understanding of the concepts, you can conquer them. By following the step-by-step approach and implementing the tips and strategies provided, you will gain the necessary skills to excel in level calculations.
Remember, level calculations are an essential part of chemistry and are used in various fields. As you progress through secondary school or college, mastering level calculations will contribute to your success in higher-level chemistry courses and future careers in the sciences.
5 out of 5
| Language | : | English |
| File size | : | 22329 KB |
| Print length | : | 99 pages |
| Lending | : | Enabled |
| Screen Reader | : | Supported |
NEW CALCULATIONS IN CHEMISTRY is a textbook specially prepared to satisfy the needs and requirements of students offering chemistry in secondary schools, as well as the candidates preparing for various categories of O' Level examinations, both local and international, for careers in the Universities, polytechnics and colleges of education.
Though the book is calculation-oriented,it is much more than that; for it is comprehensively designed to serve as a panacea for the students' problems in chemistry, especially with respect to calculations. Each topic chapter involving calculations in chemistry is thoroughly treated in details with required explanations and principles, which are highly necessary for good understanding of the subsequent calculations, and then equipped with many worked examples of international standard.
Having been involved in classroom teaching for years, as well as being also involved in various examination coordination exercises for the Examination Councils such as West African Examination Council (WAEC) and National Examination Council (NECO) in addition to the problems noted from students attempting GCSE and Cambridge International examinations, and hence knowing where the students find it most difficult in chemistry calculations, the author has therefore drawn various examination questions from various Examination Boards as samples for clarification and wide-range methods of application. They are solved in a simplified and step-by-step logical manner to ensure easy understanding and assimilation for the students and other users.
Chemistry teachers can also find the book immensely valuable for its wide coverage, explicitness and simplicity. Besides, it serves as a basic foundation for the students and candidates pursuing chemistry-related courses such as Engineering, Medicine, Natural sciences etc. as a career.
The following topics were highly treated in details: Atomic structure / Electronic configurations (in terms of normal distribution in the shells and spdf),Laws of Chemical Combination, Mole / Mole Concept (Avogadro's Constant),Gas Laws (including Gay-Lussac's Limiting and Excess reactants),Solubility (and Solubility curve) / Solubility Products, Redox reaction (including determination of Oxidation Number, Reducing and Oxidizing agents, and Balancing of Redox equations),Electrolysis (in aqueous and molten forms / calculations),Electrode Potentials (electrochemical cells),Energy diagrams (Exothermic and Endothermic reactions),Enthalpies of reactions, Spontaneity of reactions (including Free energy) and Chemical equilibrium / pH
The uniqueness of the book can never be over-emphasized; IUPAC System was embraced throughout, and besides the units of quantities were correctly dimensioned during calculations.
BEMU CHRISTIAN C.

 Howard Powell
Howard PowellUnmasking the Enigma: A Colliding World of Bartleby and...
When it comes to classic literary works,...

 Jeffrey Cox
Jeffrey CoxCritical Digital Pedagogy Collection: Revolutionizing...
In today's rapidly evolving digital...

 Quincy Ward
Quincy WardThe Diary Of Cruise Ship Speaker: An Unforgettable...
Embark on an incredible...

 Derek Bell
Derek BellBest Rail Trails Illinois: Discover the Perfect Trails...
If you're an outdoor enthusiast looking...

 Adrian Ward
Adrian WardChild Exploitation: A Historical Overview And Present...
Child exploitation is a...

 Camden Mitchell
Camden MitchellThe Untold Story Of The 1909 Expedition To Find The...
Deep within the realms of legends and...

 Spencer Powell
Spencer PowellThrough The Looking Glass - A Wonderland Adventure
Lewis Carroll,...

 Sidney Cox
Sidney CoxAdvances In Food Producing Systems For Arid And Semiarid...
In the face of global warming and the...

 Art Mitchell
Art MitchellThe Devil Chaplain: Exploring the Intriguing Duality of...
When it comes to the relationship between...

 Edgar Hayes
Edgar HayesThe Mists of Time: Cassie and Mekore - Unraveling the...
Have you ever wondered what lies beyond...

 John Steinbeck
John SteinbeckOn Trend: The Business of Forecasting The Future
Do you ever wonder what the future holds?...

 Tim Reed
Tim ReedLove Hate Hotels Late Check Out
Have you ever experienced the joy of...
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Edwin BlairYou Are Looking Live: The Iconic Catchphrase That Changed Sports Broadcasting...
Edwin BlairYou Are Looking Live: The Iconic Catchphrase That Changed Sports Broadcasting...
 Joseph HellerThe Ultimate Guide to Barefoot Skiing: Uncover the History, Tips, FAQs, and...
Joseph HellerThe Ultimate Guide to Barefoot Skiing: Uncover the History, Tips, FAQs, and...
 Hudson HayesReflections On The Legal Personhood Of Animals: Exploring the Evolving Legal...
Hudson HayesReflections On The Legal Personhood Of Animals: Exploring the Evolving Legal...
 Elmer PowellRural Development And Regime Consolidation After 1979 - How It Transformed...
Elmer PowellRural Development And Regime Consolidation After 1979 - How It Transformed... Adam HayesFollow ·8.7k
Adam HayesFollow ·8.7k Dale MitchellFollow ·17.9k
Dale MitchellFollow ·17.9k John ParkerFollow ·17.8k
John ParkerFollow ·17.8k Bryce FosterFollow ·2k
Bryce FosterFollow ·2k Jim CoxFollow ·19.5k
Jim CoxFollow ·19.5k Bradley DixonFollow ·4.1k
Bradley DixonFollow ·4.1k Brenton CoxFollow ·16.4k
Brenton CoxFollow ·16.4k Dion ReedFollow ·14.3k
Dion ReedFollow ·14.3k














